[latexpage] This past fall was my first quarter of graduate school, and one of our core courses was Deterministic Models in Biology. For our final project, we chose a quantitative biology paper on a topic of our interest and presented on it to the class. The paper I chose was a review paper, Psychiatric Illnesses as Disorders of Network Dynamics by Daniel Durstewitz, Quentin J.M. Huys, and Georgia Koppe. My undergraduate research focused on the dynamics of neurons at the molecular level, and this paper helped me connect it to specific characteristics of mental illnesses.
This paper proposes that since observable cognitive and emotional states rely on the underlying dynamics of neuronal networks, we should use Dynamical Systems Theory (DST) to characterize, diagnose, and develop therapeutic strategies for mental illness.
The central idea of DST is that there is a set of differential equations that evolve in time. A set of dynamical equations could look as follows:
\[\frac{dx_1}{dt} = \dot{x_1} = f_1(x_1, … , x_M, t; \boldsymbol{\theta} )\]
\[\frac{dx_2}{dt} = \dot{x_2} = f_1(x_1, … , x_M, t; \boldsymbol{\theta})\]
\[\vdots \]
\[\frac{dx_M}{dt} = \dot{x_M} = f_M(x_1, … , x_M, t; \boldsymbol{\theta})\]
The variables $x_1, x_2, … x_M$ represent the dynamical variables such as voltage or neural firing rate. These equations describe how each of these variables change over time. $\boldsymbol{\theta}$ represents parameters, fixed values that are properties of the system that do not change over time.
We define a fixed point as the point at which the derivatives of all of the variables are equal to 0. Fixed points are stable if activity converges towards them, and unstable if activity diverges from them. Stable fixed points are called attractors. We can define the basin of attraction as the set of points from which activity converges towards the attractor.
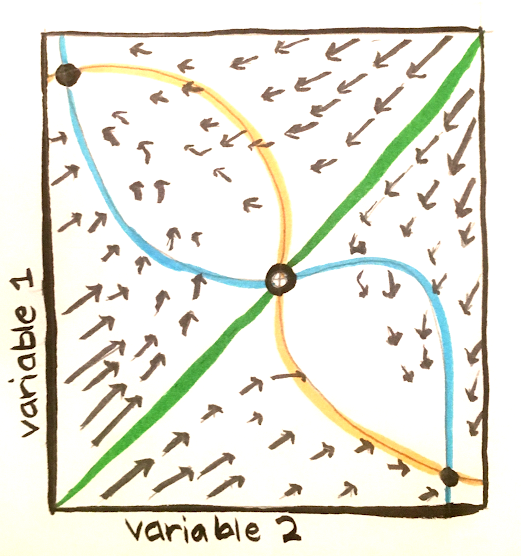
The figure below shows an example of a phase plane, a representation of a space spanned by the two variables of a system. Note that it is possible to use dimensionality reduction methods to obtain visual representations for higher dimensional systems. The arrows show the activity of the system. The blue and orange curves represent nullclines, and along each of these curves, the derivative of one of the variables is 0. The green line represents the barrier between the two basins of attractions. It is possible to cross over this barrier as a result of either external influences or random fluctuations.
I will discuss some basic neuroscience before going into the dynamics of mental illnesses. There are many ion currents that pass through a neuron membrane such as sodium, potassium, and calcium. The dynamics of these ions are driven by electrochemical gradients. Spiking activity occurs when there is a rapid influx of sodium ions, producing the spike followed by an efflux of potassium ions, returning the membrane potential to the threshold potential.
We can think of a neuron membrane as a capacitor, where positive and negative charges are accumulated on either side. The current is the rate of charge flowing per time, $I = \frac{dq}{dt}$, and the charge of a capacitor is defined as q = CV. The current through the membrane is this $ I_m = C_m \frac{dV_m}{dt}$. We can think of this system as the circuit shown below:
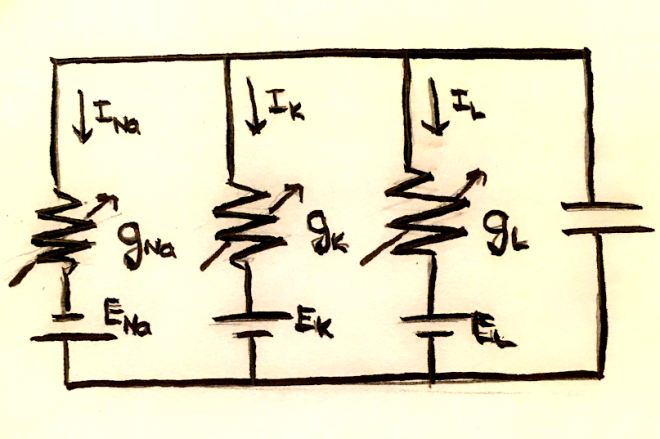 Because of charge conservation, the sum of the currents across the capacitor and each of the resistors must be 0. In mathematical terms, this is $C_m \frac{dV_m}{dt} = -\sum_i I_i$.
Because of charge conservation, the sum of the currents across the capacitor and each of the resistors must be 0. In mathematical terms, this is $C_m \frac{dV_m}{dt} = -\sum_i I_i$.
If we approximate each of these currents as ohmic, they will satisfy Ohm’s law, V = IR, meaning that the current is proportional to the difference between the membrane voltage and the threshold voltage by a factor of 1/R, or in other words, the conductance.
If the conductance were constant over time, these would be linear. However, the conductance depends on the proportion of ion channels that are open and the proportion of channels that are closed, called the gating variables. For example, a sodium current can be described as
$I_{Na} = g_{max}m^3h(V_m – E_{Na})$
In this system, m and h are the gating variables, and they vary from 0 to 1, and $g_{max}$ is the maximal conductance.
We can think of the dynamical equations for the gating variables as the result of a mass equation. Consider the reaction
$Closed \rightleftharpoons Open $
Suppose $\alpha$ is the rate of opening of a channel, or the forward reaction above, and $\beta $ is the rate of closing, the reverse reaction above, and both of these rates depend on the voltage. If m represents the proportion of channels that are open, the derivative over time is equal to the forward rate times the concentration of reactants minus the reverse rate times the concentration of products. In other words:
$\frac{dm}{dt} = \alpha(V_m)(1-m) – \beta (V_m)m$
Another form of this dynamical equation commonly seen in the literature is:
$\frac{dm}{dt} = \frac{m_{\infty}(V_m) – m}{\tau_{Na}(V_m)}$
$\tau_{Na}$ is the voltage-dependent time constant, and $m_{\infty}$ is the steady-state proportion of open channels as a function of voltage.
The dynamical equation for voltage for the simple NaKL model is as follows:
\[\frac{dV}{dt}
= g_{Na}m^3h(E_{Na}-V) + g_K n^4 (E_K -V) + g_L (E_L – V) + I_{inj}C^{-1}\]
Neuronal networks are the result of multiple neurons connected to one another through synapses. Pre-synaptic neurons deliverer chemicals, called neurotransmitters, to post-synaptic neurons. Some neurotransmitters are excitatory, such as NMDA (N-Methyl-D-aspartic acid), meaning they increase the likelihood of spiking activity, and others are inhibitory, such as GABA (gamma-aminobutyric acid), meaning that they decrease the likelihood of spiking activity. To describe the networks of neuronal networks, each individual neuron has a voltage equation as illustrated above, with additional terms relating to its synaptic currents. These currents depend on the synaptic conductance, the difference between the membrane voltage and the threshold voltage, the strengths of the synaptic connections, and the fraction of open channels for each receptor. The dynamical equation for the fraction of open channels usually depends on properties of the presynaptic neuron.
So far, the variables we have been considering have been the voltage and the gating variables. In order to discuss the dynamics of mental illness, we must think about another important variable: firing rate. This simply describes the rate of voltage spikes over time. Below is an example of a phase plane, where the vertical axis is the average firing rate of inhibitory neurons, and the horizontal axis is the average firing rate of excitatory neurons.
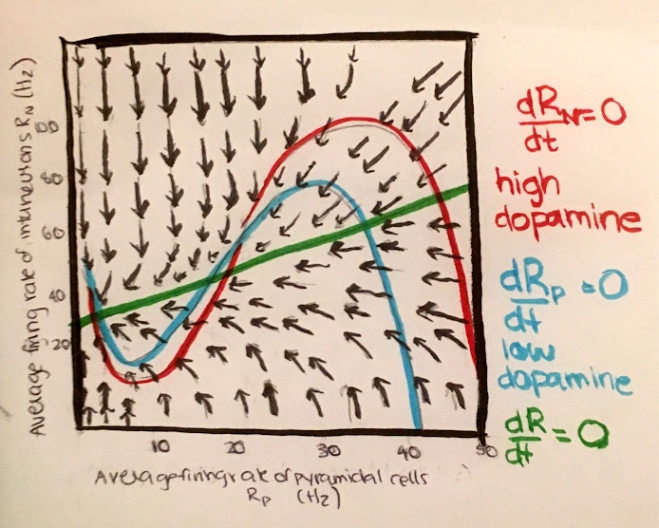
In this system, the fixed points can be thought of as memories or goal-states, and we can use this system to consider the effects of the underlying dynamics on working memory or decision making. Increasing the depth of the basin of attraction can have the effect of increasing the stability of the state, while flattening the basin of attraction reduces the stability of the state.
This paper highlights the key role of dopamine in altering these attractor dynamics. Stimulating the D1 dopamine receptors has the effect of increasing firing activity of both excitatory (NMDA) and inhibitory (GABA) neurons. This alters the parameters of the system, in particular, the strengths of synaptic connections, over time. As a result, the basins of attraction are deepened, and the state is more stable and robust to external perturbations or noise fluctuations.
Stimulation of the D2 dopamine receptors has the opposite effect, flattening the basins of attractions. These flat attractor landscapes could lead to disorganized or spontaneous thoughts that can be experienced as hallucinations that are characteristic of schizophrenia. This can also explain the high distractibility in attention-deficit hyperactive disorder (ADHD). On the other hand, Obsessive Compulsive Disorder (OCD), a disorder characterized by rumination, invasive and recurrent obsessions and compulsions, can be linked to deep basins of attractions that are robust to potential distractors. Major Depressive Disorder characterized by a coexistence of rumination and a negative mood with lack of concentration and distractibility, and one can think of it as an imbalance between multiple attractor states.
The main point this review paper aims to illustrate is that in order to characterize and develop treatments for mental illnesses, one must consider the underlying network dynamics. The suggested role of dopamine in altering the depth of basins of attractions suggest that we might try to target the dynamics of schizophrenia patients, for example, through dopaminergic drugs.
I found the process of reading this review paper and the sources it cited extremely helpful for me in improving my understanding of neurons, neuronal networks, biophysics, and nonlinear dynamics, and linking my previous understanding of neurons to cognitive processes, something that I had not fully understood before. Because the review paper goes over the general information, I read many of the papers it cited to find the basis behind some of its claims. However, I still do not clearly understand the mechanism behind the changes in the attractor dynamics. I would like to learn more about how the parameters are changed, and how these changes, in turn, alter the attractor landscapes.
At this point, I believe that the connection between these dynamics and mental illnesses as presented in this paper seems rather speculative. However, I think that as more data is collected and analyzed, and further models are developed to understand the dynamics of neuronal networks, we can glean more insight towards understanding and developing treatments for mental illnesses.
References:
Durstewitz, D., Huys, Quentin J. M., Koppe, Georgia. (2018). Psychiatric Illnesses as Disorders of Network Dynamics. doi: https://arxiv.org/pdf/1809.06303.pdf
Durstewitz, D. (2009). Implications of synaptic biophysics for recurrent network dynamics and active memory. Neural Networks, 22(8), 1189-1200.
Durstewitz, D., Seamans, J. K. (2008). The dual-state theory of prefrontal cortex dopamine function with relevance to catechol-o-methyltransferase genotypes and schizophrenia. Biological Psychiatry, 64(9), 739-749.
Durstewitz, D. (2006). A few important points about dopamine’s role in neural network dynamics. Pharmacopsychiatry, 39(S 1), 72-75.
Izhikevich, E. M. (2007). Dynamical Systems in Neuroscience: MIT Press.
Johnston, Daniel, Wu, Samuel Miao-Sin . (2001) Foundations of Cellular Neurophysiology. MIT Press.
Rolls, E. T., Loh, M., Deco, G. (2008). An attractor hypothesis of obsessive-compulsive disorder. European Journal of Neuroscience, 28(4), 782-793. doi: 10.1111/j.1460-9568.2008.06379.x
Strogatz, S. H. (2018). Nonlinear dynamics and chaos: with applications to physics, biology, chemistry, and engineering: CRC Press.

